Open letter on urgent need for compassionate use of bedaquiline for XDR-TB and pre-XDR-TB in South Africa
Open letter to the Medicines Control Council and Ministry of Health on urgent need for compassionate use of bedaquiline for XDR-TB and pre-XDRTB in South Africa.
Dear Professor Eagles, Ms. Hela and Honorable Dr. Motsoaledi,
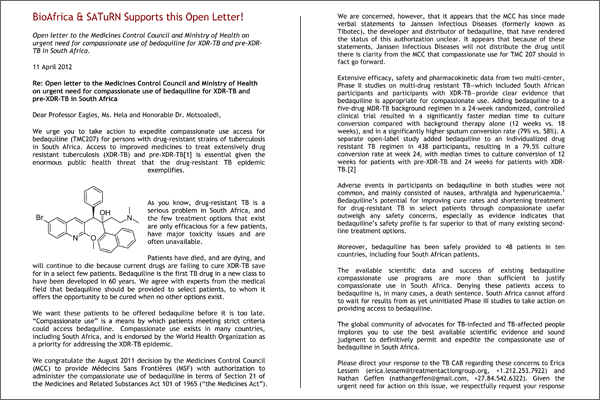
We urge you to take action to expedite compassionate use access for bedaquiline (TMC207) for persons with drug-resistant strains of tuberculosis in South Africa. Access to improved medicines to treat extensively drug resistant tuberculosis (XDR-TB) and pre-XDR-TB[1] is essential given the enormous public health threat that the drug-resistant TB epidemic exemplifies.
As you know, drug-resistant TB is a serious problem in South Africa, and the few treatment options that exist are only efficacious for a few patients, have major toxicity issues and are often unavailable. Patients have died, and are dying, and will continue to die because current drugs are failing to cure XDR-TB save for in a select few patients. Bedaquiline is the first TB drug in a new class to have been developed in 60 years. We agree with experts from the medical field that bedaquiline should be provided to select patients, to whom it offers the opportunity to be cured when no other options exist.
News date: 2012-04-11
Links:
http://www.msf.org.za/publication/open-letter-medicines-control-council-south-africa
KRISP has been created by the coordinated effort of the University of KwaZulu-Natal (UKZN), the Technology Innovation Agency (TIA) and the South African Medical Research Countil (SAMRC).
Location: K-RITH Tower Building
Nelson R Mandela School of Medicine, UKZN
719 Umbilo Road, Durban, South Africa.
Director: Prof. Tulio de Oliveira




